Hydrogen peroxide (H2O2) is a widely used, green, and efficient oxidizing agent with increasing market demands. However, the traditional anthraquinone (AQ) oxidation production process is generally plagued by high energy consumption, substantial pollutant emissions, and complex procedures. In addition, the produced high concentration H2O2 solution poses potential safety risks during storage and transportation. By comparison, photocatalytic H2O2 production offers a green, safe, and sustainable method for on-demand production of low-concentration H2O2. Nevertheless, current photocatalytic reactions are predominantly conducted in small-volume, batch reactors, which suffer from issues such as light scattering and mass transfer limitations, especially when enlarging reaction volumes. As a result, these issues lead to low H2O2 yield and production rates, failing to meet practical application requirements. Furthermore, these reaction systems require repeated catalyst recovery and product separation operations, which are time-consuming and add extra costs. Therefore, optimizing the photocatalytic reaction system to achieve continuous H2O2 photosynthesis is essential for advancing the practical application of this technology.
To address these challenges, Professor Yanguang Li and Associate Professor Wei Huang from the Institute of Functional Nano & Soft Materials (FUNSOM) at Soochow University designed a novel biphasic flow photocatalytic reaction system. This system enables uninterrupted H2O2 synthesis along with automatic product extraction and catalyst recycling. To meet the requirements of this reaction system, the authors first synthesized a perfluoroalkyl-functionalized covalent organic framework (PF-BTTA-COF) photocatalyst, which exhibits excellent superhydrophobic properties and high photocatalytic activity and stability for H2O2 production. Based on this, the oil phase containing the photocatalyst and water were respectively pumped into a microtube reactor to form alternating oil-water biphasic micro-segments. Under illumination, H2O2 produced in the oil phase will spontaneously migrate to and accumulates in the water phase through the oil-water interface. Subsequently, in the collector, the H2O2 aqueous solution and oil phase dispersion undergo phase separation, enabling immediate product extraction and catalyst recycling. By optimizing reaction parameters, the H2O2 production rate could reach up to 968 μmol h−1, which is 1-2 orders of magnitude higher than those of traditional systems. Moreover, the obtained H2O2 concentration was tunable within the range of 2.2-38.1 mM. Remarkably, the reaction system was demonstrated a good stability in a continuous 100-hour test, ultimately producing 6 liters of 5.7 mM H2O2, which could satisfactorily meet the general demand for sterilization and wastewater treatment. Moreover, techno-economic analysis indicated that this reaction system has significant economic benefits in an industry-scale H2O2 photosynthesis. Overall, this study provides an economically feasible, green, and efficient alternative for continuous photocatalytic H2O2 production. The related results have been published in Nature Communications (Nat. Commun. 2024, 15, 8023).
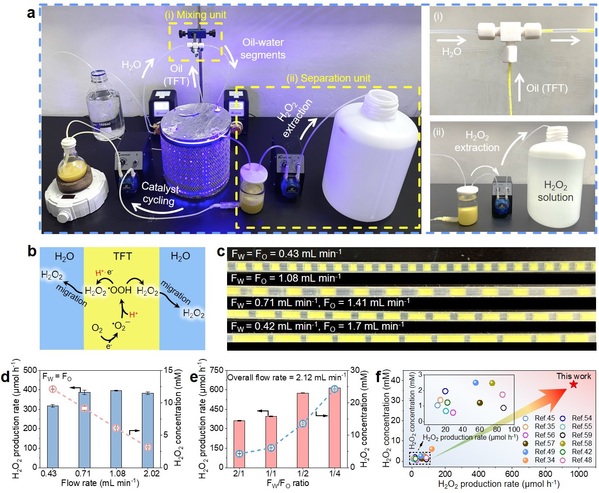
Fig 1. Photocatalytic H2O2 production in the biphasic fluid system.
Link to paper: https://www.nature.com/articles/s41467-024-52405-3
Title: Perfluoroalkyl-modified covalent organic frameworks for continuous photocatalytic hydrogen peroxide synthesis and extraction in a biphasic fluid system
Authors: Chaochen Shao, Xiaohan Yu, Yujin Ji, Jie Xu, Yuchen Yan, Yongpan Hu, Youyong Li, Wei Huang* & Yanguang Li*
Link to Prof. Yanguang Li's group: https://www.ligroup.com.cn/
Editor: Danting Xiang, Xin Du