As green renewable catalysts that have received widespread attention, non-metallic catalysts have shown importance, especially in terms of current energy and environmental challenges. Recent studies have shown that carbon-based non-metallic catalysts have significant potential for reducing costs, improving efficiency and stability, and exhibit enhanced electronic conductivity, tunable structures, and good adaptability to different acid‒base environments. Its catalytic performance is significantly affected by factors such as porosity, functional groups, specific surface area, π conjugated system, donor‒acceptor structure, and heteroatom doping. Moreover, hydrogen peroxide (H2O2), an important environmentally friendly oxidant, uses solar energy for photocatalysis in its production process and is regarded as an efficient and environmentally friendly alternative. Although many studies have investigated the reaction mechanism of the photocatalytic synthesis of H2O2, owing to the complexity of the carbon-based catalyst structure, the specific active sites are still unclear, leading to controversy over the mechanism of the reaction, which makes improving high-performance non-metallic catalysts that still rely on a trial-and-error process difficult, thus, research in this area faces many challenges.
In response to the above challenges, the teams of Prof. Zhenhui Kang, Prof. Yang Liu and Prof. Tao Cheng from the Institute of Functional Nano and Soft Materials (FUNSOM) of Soochow University recently proposed the design of a photocatalyst with a known small molecule structure (EDTA) to understand the photolysis of water in detail to produce H2O2. First, EDTA was polymerized into non-metallic materials with different structures by controlling the polymerization temperature. Using different characterization methods, the changes in the structural characteristics of the materials in different temperature ranges were revealed: when the polymerization temperature is lower than 240 °C, the material maintains the original structure of EDTA; when the polymerization temperature is range of 250 °C to 350 °C, the material has a characteristic structural unit similar to N-ethyl 2-piperazinone, and many conjugated carbonyl structures are present in this unit; when the polymerization temperature exceeds 400 °C, the saturated carbon linker and amide structure of the material are destroyed, and the material polymerizes to form an aromatic π-conjugated system, thus resulting in a similar structure of graphitic carbon rings. In addition, insitu experiments and theoretical calculations revealed that the conjugated carbonyl oxygen in EA-260 is the key active site for photocatalytic H2O2 production. The results of the photocatalytic design experiments with different concentrations of water and numbers of electrons transferred by electrochemical oxygen reduction indicate that there are cascade reaction pathways of the water-oxygen reduction reaction (WOR) and the oxygen reduction reaction (ORR) in the catalytic system. In addition, the photocatalytic results of more small-molecule structures further verified the unique activity of the conjugated carbonyl structure for the photocatalytic production of H2O2. This work is crucial for elucidating how carbon-based materials provide reaction sites and regulate the microenvironment of local reactions in certain photocatalytic reactions, which provides ideas and methods for understanding the structural selection of non-metallic materials. Relevant results have been published in the Journal of Nature Communications (DOI: 10.1038/s41467-024-52162-3).
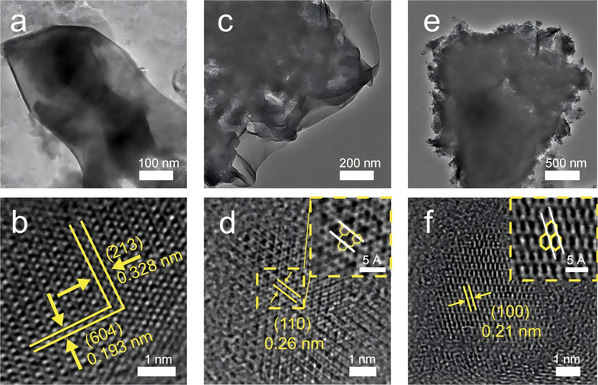
Fig 1. Surface morphology of synthesized carbon-based materials with different structures
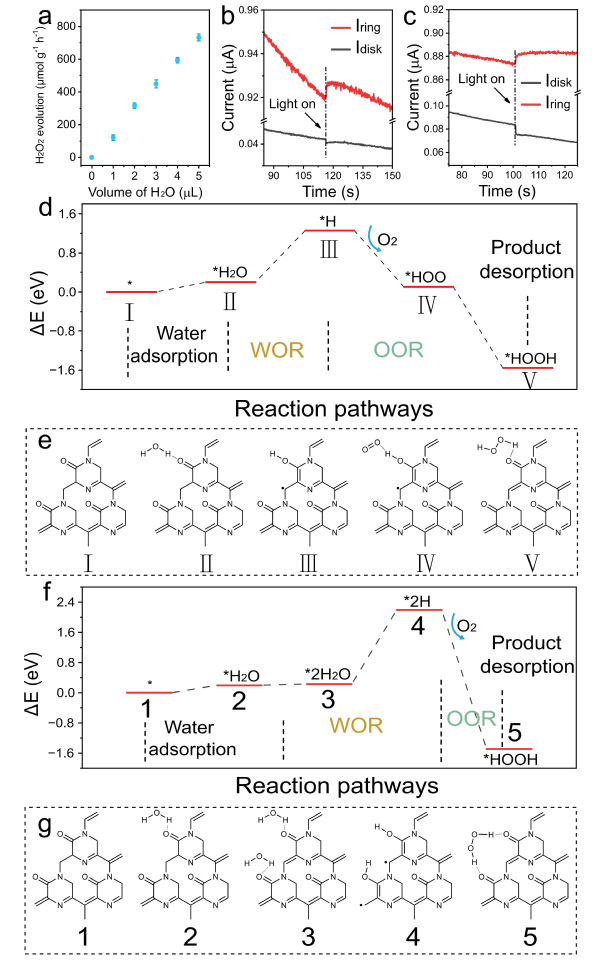
Fig 2. Study on the mechanism of photocatalytic H2O2 production.
Link to paper: https://doi.org/10.1038/s41467-024-52162-3
Title: A metal-free cascaded process for efficient H2O2 photoproduction using conjugated carbonyl sites
Authors: Tiwei He, Hongchao Tang, Jie Wu, Jiaxuan Wang, Mengling Zhang, Cheng Lu, Hui Huang, Jun Zhong, Tao Cheng*, Yang Liu* & Zhenhui Kang*
Link to Prof. Zhenhui Kang's group: http://nano.suda.edu.cn/kanggroup
Editor: Danting Xiang, Xin Du